【互联网 www.tofms.org】 【My Blog】
如需转载本站内容,请注明资料来源于: www.tofms.org
|
| 【技术】【释疑】美国疫苗NVX-CoV2373的技术核心 |
这两天,川某某又吹了,美国国防部投资疫苗了。这是个什么疫苗呢?本人快速追踪了一下,看下面的描述。
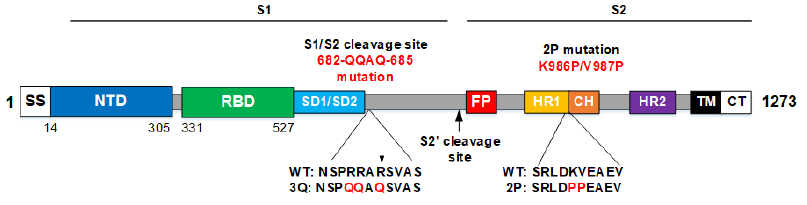 • Seasonal InfluenzaNanoFlu Nanoparticle Vaccine (qNIV) • Nanoparticle and Matrix M adjuvant share many similarities with NVX‐CoV237 • SARS CoV‐2 Spike (S) gene synthesized by GenScript and delivered Jan 20 • Engineered >20 constructs. • Screened for hACE2‐binding, stability,productivity, immunogenicity. • Developed from a gene sequence to phase 1 in ~90 days nanoparticle vaccines against Ebola GP and influenza A/H7N9 HA with Matrix M adjuvant 皂苷(saponin). 【3】Matrix M adjuvant(saponin皂苷佐剂) Matrix-M is a saponin-based adjuvant consisting of two populations of individually formed 40 nm sized Matrix particles, each with a different and well-characterized saponin fraction with complementary properties ( Fraction-A and Fraction-C, respectively). Matrix-M, used in this study, consists of 85% Matrix-A and 15% Matrix-C. The Matrix particles are formed by formulating purified saponin from Q. saponaria Molina with cholesterol and phospholipid . 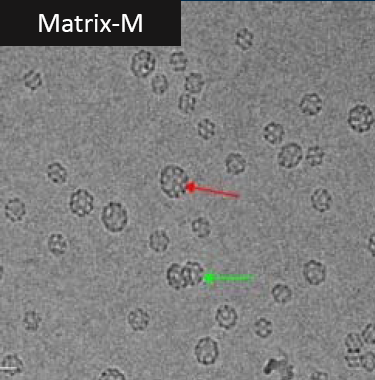 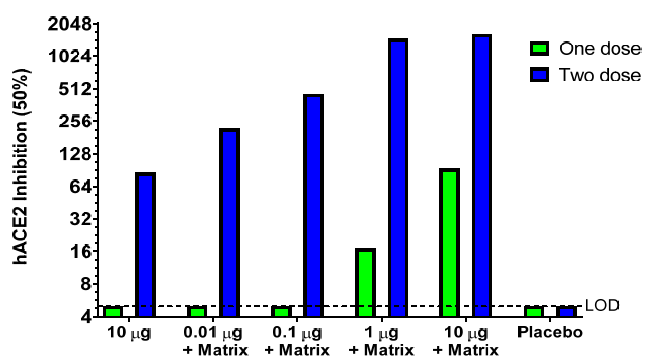 更多精彩文章,请关注公众号 【药网堂】 All rights reserved , visit the micromessage 药网堂 for more @ tofms_org@126.com  【wkh, 2020-06-07 19:14:58】 【责任人 wkh】 [已阅读 1941 次] |
Email:tofms_org@126.com 【有疑问,发邮件】 © 2008- All Rights Reserved, Powered by WKH© 2008 |